The Colospan Device: CG-100 Clinical Study
The CG-100 is being evaluated as an alternative to ostomy bags for patients with colorectal cancer
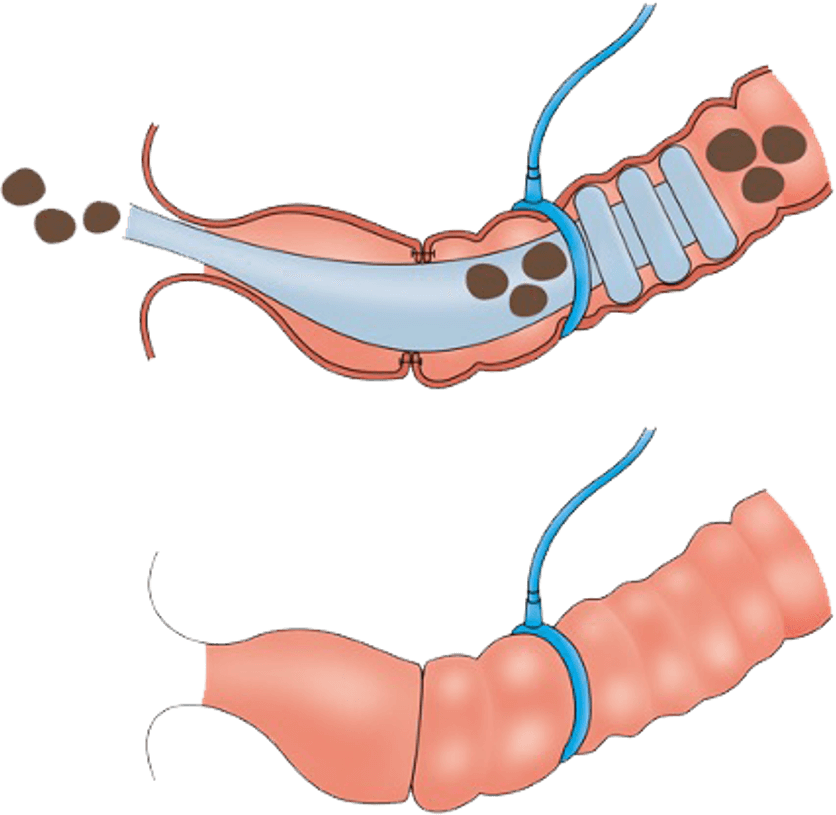
As a patient with colorectal cancer, your oncologist and surgeon may recommend surgery to remove the cancerous part of your bowel. A dangerous complication of this surgery is internal leakage of digestive waste where the two healthy ends of your bowel are surgically reconnected. As a result, an ostomy bag is often used to temporarily divert the waste outside the body from an opening in your abdomen. In recognition that living with an ostomy bag can present daily challenges that can affect overall health and quality of life for colorectal patients, an alternative has been developed and is being evaluated in the CG-100 clinical study.